/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/909bec1c-1f4c-11ef-9876-0242ac120013/1-1-image-biosimilaires.png)
Biosimilars: Opportunities and Challenges for the Pharmaceutical Industry
With the recent and imminent arrival of numerous biosimilars on the market, the pharmaceutical industry is undergoing rapid change. These drugs, equivalents of original biologics whose patents have expired, present numerous opportunities, but also considerable challenges for the various players in this market. We aim to explore in an impartial manner the growing importance of biosimilars, their adoption, as well as the regulatory and commercial obstacles they face.
Growing importance of biosimilars
Biosimilars, biological products similar to approved drugs (reference products), are playing an increasingly crucial role in the pharmaceutical sector. Their importance increases as patents on original biological drugs expire, paving the way for sometimes more affordable alternatives. This dynamic is transforming the pharmaceutical landscape and offering significant opportunities for healthcare systems and patients.
Substantial savings for health systems
One of the main advantages of biosimilars is their ability to generate substantial savings . By reducing the costs of biological treatments, they allow healthcare systems to reallocate resources to other critical areas. A study by the European Medicines Agency (EMA) shows, for example, that the introduction of biosimilars in Europe has led to savings of up to 33% compared to the costs of reference biological medicines . ( Le Moniteur des pharmacie.fr ).
Improving access to treatment
Biosimilars also play an important role in improving patient access to treatments . The high costs of biologic medicines can limit access for many patients, particularly in low- and middle‑income countries without obvious access to social security. By offering lower‑cost alternatives, biosimilars enable more patients to benefit from lifesaving treatments . The World Health Organization (WHO) actively supports the adoption of biosimilars to increase access to medicines and improve global health outcomes ( Pfizer France ).
Impact on innovation and competition
The arrival of biosimilars on the market also appears to be an excellent stimulus for innovation and competition in the pharmaceutical sector. Biotechnology companies are incentivized to develop new solutions and improve existing drugs to maintain their competitive advantage. This dynamic benefits patients, as it leads to more innovative and effective treatments.
Adoption of Biosimilars
Biosimilar adoption is progressing rapidly worldwide, although the pace and extent of their integration vary considerably across regions and healthcare systems. Several factors influence this adoption, including government policies, reimbursement strategies, and healthcare professional and patient perceptions.
Adoption in Europe
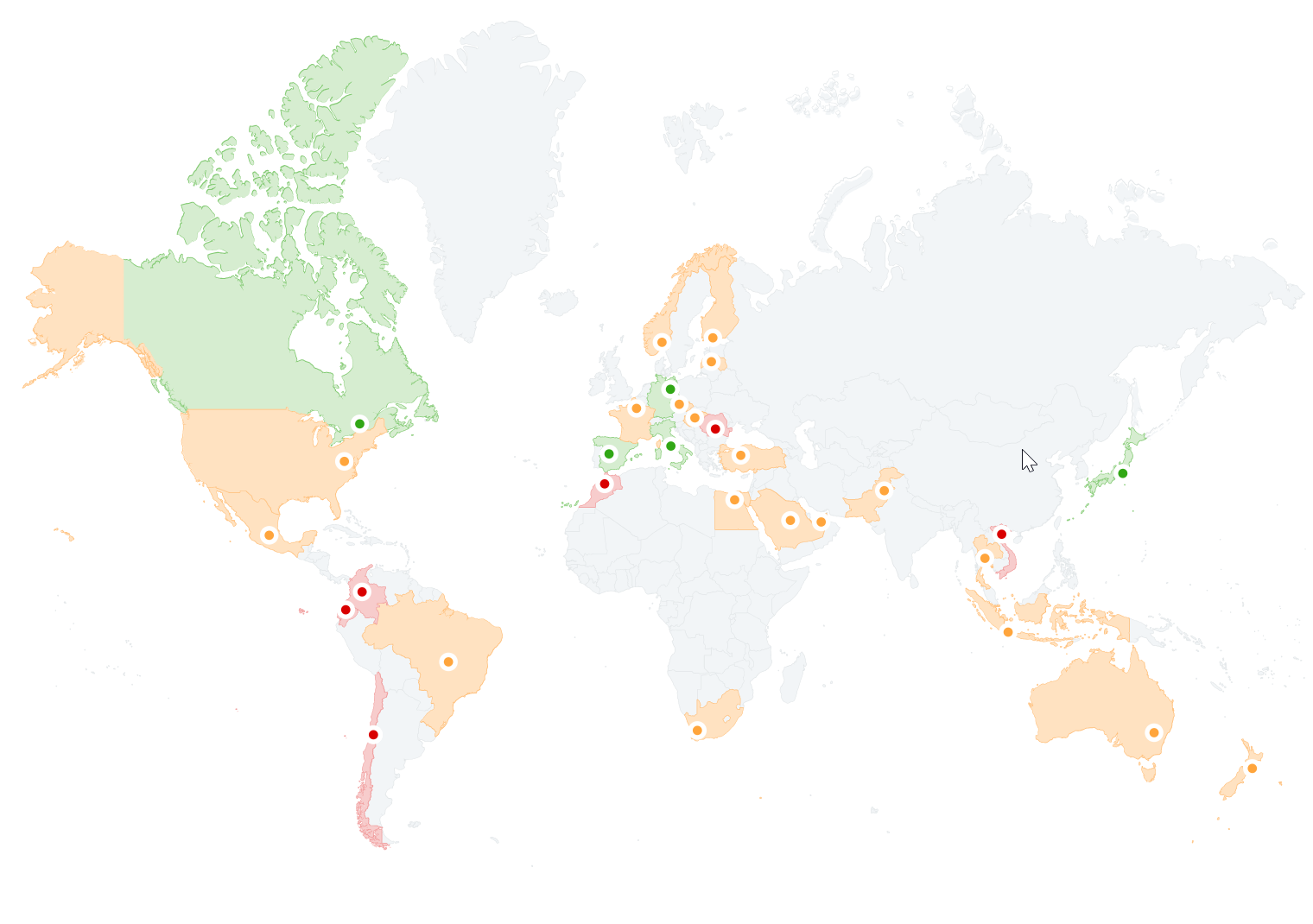
Europe is often cited as a model for biosimilar adoption. Since the EMA approved the first biosimilar in 2006, the European biosimilar market has grown steadily. Currently, more than 60 biosimilars have been approved in the European Union , covering a variety of treatments ranging from oncology to rheumatology ( Le Moniteur des pharmacien.fr ). .
Supportive policies and government initiatives obviously play a crucial role in this adoption. For example, several European countries, such as Germany and the United Kingdom, have implemented incentive programs to promote the use of biosimilars . These programs include financial incentives for prescribers, patient education campaigns, and favorable reimbursement strategies.
Map of biosimilar adoption around the world, click on the image to access the study.
Adoption in the United States
In the United States, the biosimilar market is newer and its adoption has been slower compared to Europe . Since the FDA approved the first biosimilar in 2015, the number of approved biosimilars has exceeded 30. Several obstacles appear to have hampered their adoption, including patent disputes , pay‑for‑delay strategies , and uncertainties regarding reimbursement policies. ( Pharmaceuticals ).
Despite these challenges, the situation is changing. Recent policy reforms and FDA initiatives aim to accelerate the approval of biosimilars and encourage their use. Payers and healthcare providers are beginning to recognize the economic benefits of biosimilars , which should boost their adoption in the future. .
Perception of healthcare professionals and patients
The perception of biosimilars by healthcare professionals and patients is a key factor in their adoption. Studies show that initial mistrust of the efficacy and safety of biosimilars decreases as clinical experience accumulates and scientific evidence strengthens . Information campaigns and training programs for healthcare professionals play an important role in dispelling reluctance and promoting adoption. .
For patients, acceptance of biosimilars often depends on effective communication from healthcare professionals about their safety and efficacy. Outreach and education initiatives for both patients and healthcare professionals are essential to building trust in these treatments. .
Future prospects
With the expiration of many leading biologic drug patents, the adoption of biosimilars is poised to grow. This momentum is fueled by concerted efforts by regulators, payers, and manufacturers to overcome current barriers and promote the benefits of biosimilars in an effort to address supply issues. The continued development of favorable policies and support programs promises to further facilitate the integration of biosimilars into clinical practice and maximize their benefits for healthcare systems and patients.
Regulatory challenges
Biosimilars, while offering significant benefits in terms of cost reduction and improved access to treatments, face significant regulatory challenges. These challenges stem from the inherent complexity of producing these biologics and the stringent requirements imposed by health authorities to ensure their safety and efficacy . Here is an overview of the main regulatory challenges facing biosimilars.

Marketing Authorization Requirements
One of the first barriers for biosimilars is obtaining marketing authorization (MA) . Unlike traditional generic drugs, which are exact chemical copies of their brand‑name counterparts, biosimilars are complex biological products. As a result, the requirements for proving their equivalence in terms of safety, efficacy, and quality are much higher. Manufacturers must conduct extensive comparative studies, including clinical trials , to demonstrate that their product is highly similar to the reference product ( Le Moniteur des pharmacien.fr ).
In Europe, the EMA has established strict guidelines for the evaluation of biosimilars, requiring robust data from preclinical and clinical testing . Similarly, the FDA in the United States imposes rigorous criteria for the approval of biosimilars , although approaches may differ slightly between the two agencies, creating additional challenges for companies seeking multiple approvals ( Pfizer France ).
Variability in international regulations
Another major challenge is the variability of international regulations. Each region or country may have specific requirements for the approval of biosimilars , which complicates the market launch strategy for manufacturers. For example, some regions may require additional studies or specific types of clinical data , making the process more costly and time‑consuming ( Pharmaceuticals ).
This regulatory heterogeneity can also lead to delayed launch times, limiting rapid patient access to treatments . Companies must navigate a complex regulatory landscape and adapt to local requirements for each target market.
Demonstration of similarity and interchangeability
Biosimilars need to demonstrate their similarity to the reference product and, in some cases, their interchangeability. Similarity must be proven through extensive quality, safety, and efficacy studies . Interchangeability, which allows the biosimilar to be substituted for the reference product without prior consultation with the prescriber, is not automatically granted and requires additional evidence . In the United States, for example, the FDA has so far granted interchangeability status to only a limited number of biosimilars. .
Post‑market surveillance
Post‑market surveillance (pharmacovigilance) helps ensure the continued safety of biosimilars. Regulatory authorities require robust pharmacovigilance plans to detect, assess, and prevent potential adverse events. This surveillance is particularly important for biosimilars, as even minor differences in manufacturing processes can have significant impacts on product safety and efficacy.
Development costs and time
Biosimilar development is costly and time‑consuming. The clinical trials and comparative studies required to meet regulatory requirements represent a significant financial investment . Additionally, the time required to obtain approvals can delay market launch, impacting companies' profitability. According to a report by the Pharmaceutical Research and Manufacturers of America (PhRMA), the cost of developing a biosimilar could reach hundreds of millions of dollars. .

Business Challenges
The adoption of biosimilars in the pharmaceutical market faces several commercial constraints. These obstacles can hamper their deployment and affect the profitability of the companies that produce them. Here's a detailed look at these challenges.
Education and awareness of health professionals
Awareness and education among healthcare professionals are essential for the adoption of biosimilars. However, there is some mistrust and reluctance to use these products due to the complexity of their development and the perceived differences from reference products . Biosimilar manufacturers must invest heavily in educational programs and awareness campaigns to convince prescribers of the safety and efficacy of their products. .
Training initiatives should be ongoing and tailored to address the specific concerns of healthcare professionals . For example, online training sessions, webinars, and seminars can be organized to provide clinical evidence and comparative data between biosimilars and reference products.
Refund policies and market access
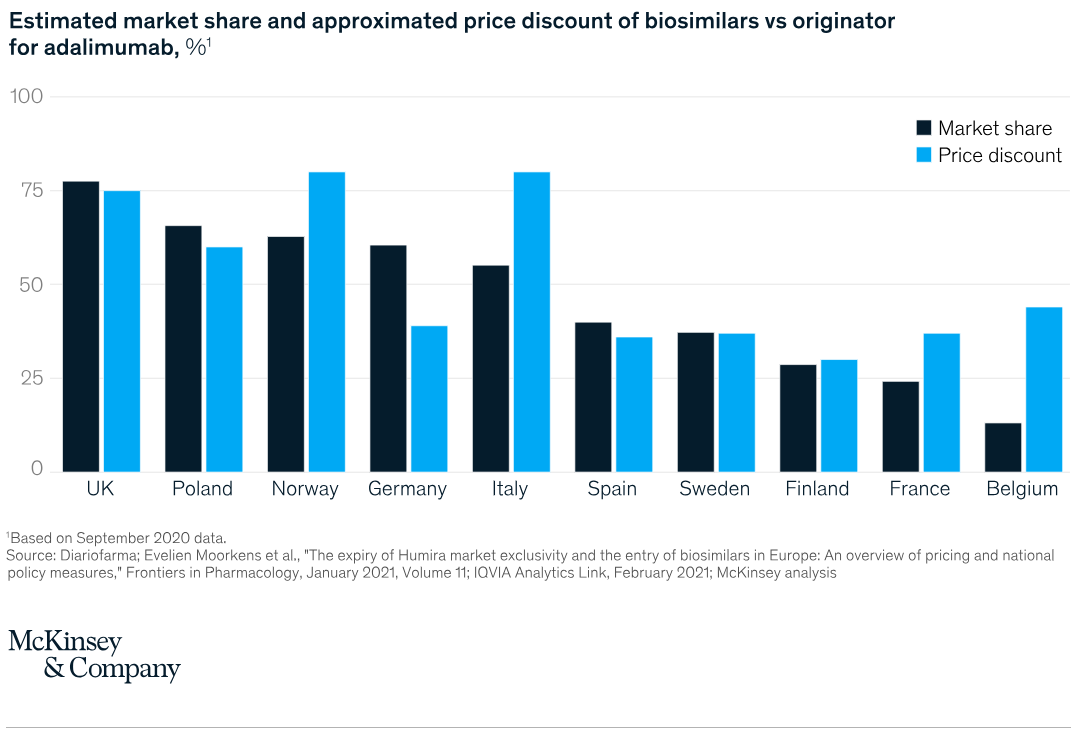
Reimbursement policies vary considerably between countries and can strongly influence the adoption of biosimilars. In some markets, biosimilars may not benefit from the same level of reimbursement as reference products , making their adoption less attractive to patients and prescribers. Biosimilar manufacturers must navigate these complex reimbursement policies and work with health authorities to ensure their products are included in the lists of reimbursable medicines ( Pfizer France ).
Comparison of Biosimilar Reimbursement Policies Across the Globe - McKinsey & Company Study - Access the study by clicking on the image.
Production costs and profit margins
Biosimilar production is a costly and complex process. Biosimilars require significant investments in research, development, and manufacturing . Companies must set up high‑tech production facilities and adhere to rigorous quality standards . These high costs can reduce profit margins and make it difficult to compete on price with reference products and other biosimilars. ( Press - Ministry of Finance ).
Patient adoption
Finally, patient adoption also appears to be a significant barrier for players in this market. Patients' perception of biosimilars can affect their acceptance. Patients may be reluctant to switch treatments due to concerns about the safety and efficacy of biosimilars . Companies must therefore invest in awareness campaigns and provide clear and convincing information to gain patient trust ( Le Moniteur des pharmacie.fr ) .
Despite numerous regulatory and commercial challenges, the biosimilar industry continues to grow, driven by the demand for more affordable and accessible treatments. Companies that successfully navigate this complex landscape can not only benefit from new markets, but also significantly contribute to improving healthcare globally . Close collaboration with regulatory authorities and innovation in development processes are essential to overcome these obstacles and maximize the potential of biosimilars.
Find a strong ally in Pharmafield
Whether you are a biosimilar developer or a reference product supplier, Pharmafield is here to help you overcome these challenges. We understand the regulatory complexities, high development costs, and market defense strategies that can impact your success.
Are you experiencing challenges with your biosimilars or reference products? Let us help. Send us an email and we'll get back to you within 2 hours.
Pharmafield is committed to providing customized solutions to maximize the potential of your products, whether biosimilars or reference medicines, and ensure their success in the marketplace. Our expertise is at your service to support you in every step of your commercial strategy.
:strip_exif()/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/0f7612d6-5974-11ea-a4ee-0242ac130005/1-1-logo.png)
:strip_exif()/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/0f7612d6-5974-11ea-a4ee-0242ac130005/1-1-logo.png)
:strip_exif()/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/364cce60-d650-11ee-bead-0242ac12000a/1-1-pharmaceutiques-logo.png)
/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/65756118-6985-11ed-ad9a-0242ac14000b/1-1-2001-i126-022-medical-conference-set-05-jpg.png)
:strip_exif()/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/b2360efc-e43d-11ed-a055-0242ac140005/1-1-1-image-video-11-04-23.png)
:strip_exif()/reboot/media/4a8df14a-8cc2-11f0-9adc-1a3d8e5696a4/4d3fc3e8-67db-11f0-8984-365b7e6e4d74/1-1-picture-petit-of-attractive-saleswoman-on-meeting-in-off-2023-11-27-05-36-18-utc-copie.jpg)